Pioneering Industrial-Scale Exosome Production For Tomorrow’s Medicine
Unlocking the Therapeutic Potential of Nature’s Nanoscale Messengers
Are you looking for exosome bioprocess development or production monitoring ?
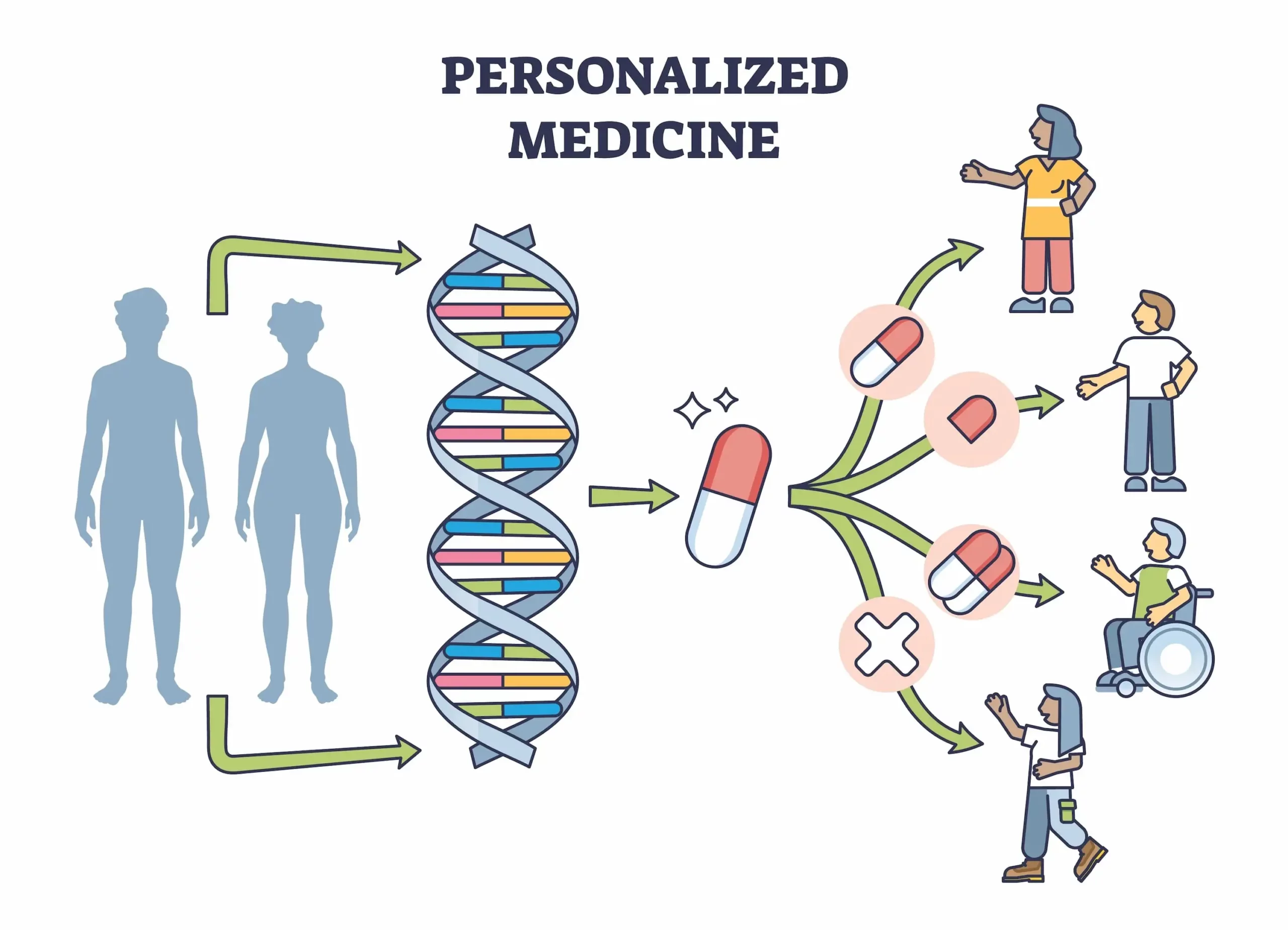
Moved from systemic to precision
Therapeutic development has progressively moved away from systemic, broad-spectrum approaches toward targeted precision delivery enabling therapeutic molecules to reach specific tissues, penetrate cells effectively, and regulate disease mechanisms while minimizing off-target toxicity
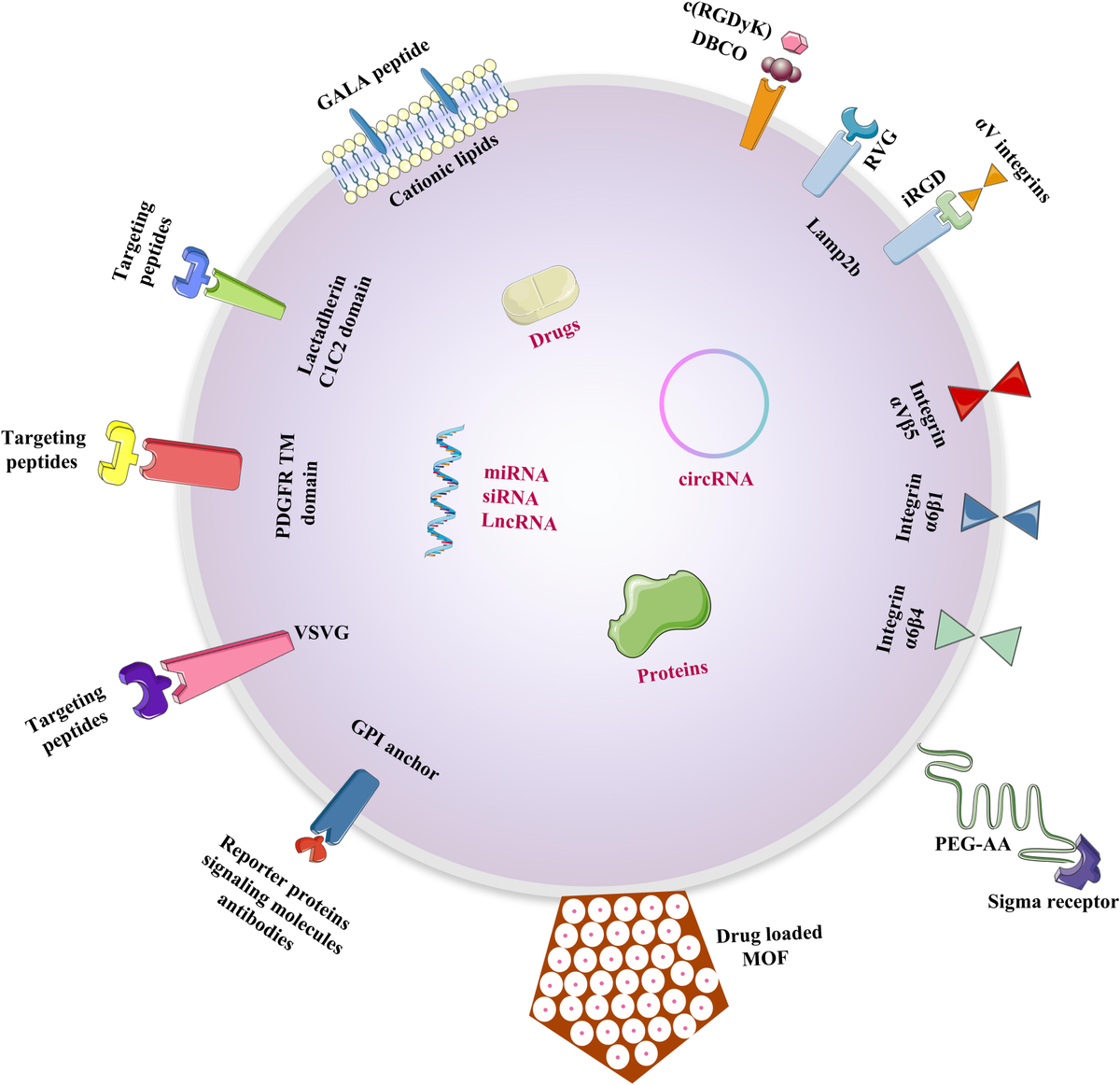
Therapeutic potential
Exosomes’ therapeutic potential stems from their unique delivery platform rather than their payload composition. Their endogenous origin, immunological compatibility, and intrinsic cellular uptake mechanisms enable them to overcome limitations commonly encountered with synthetic nanoparticles and viral vector systems.
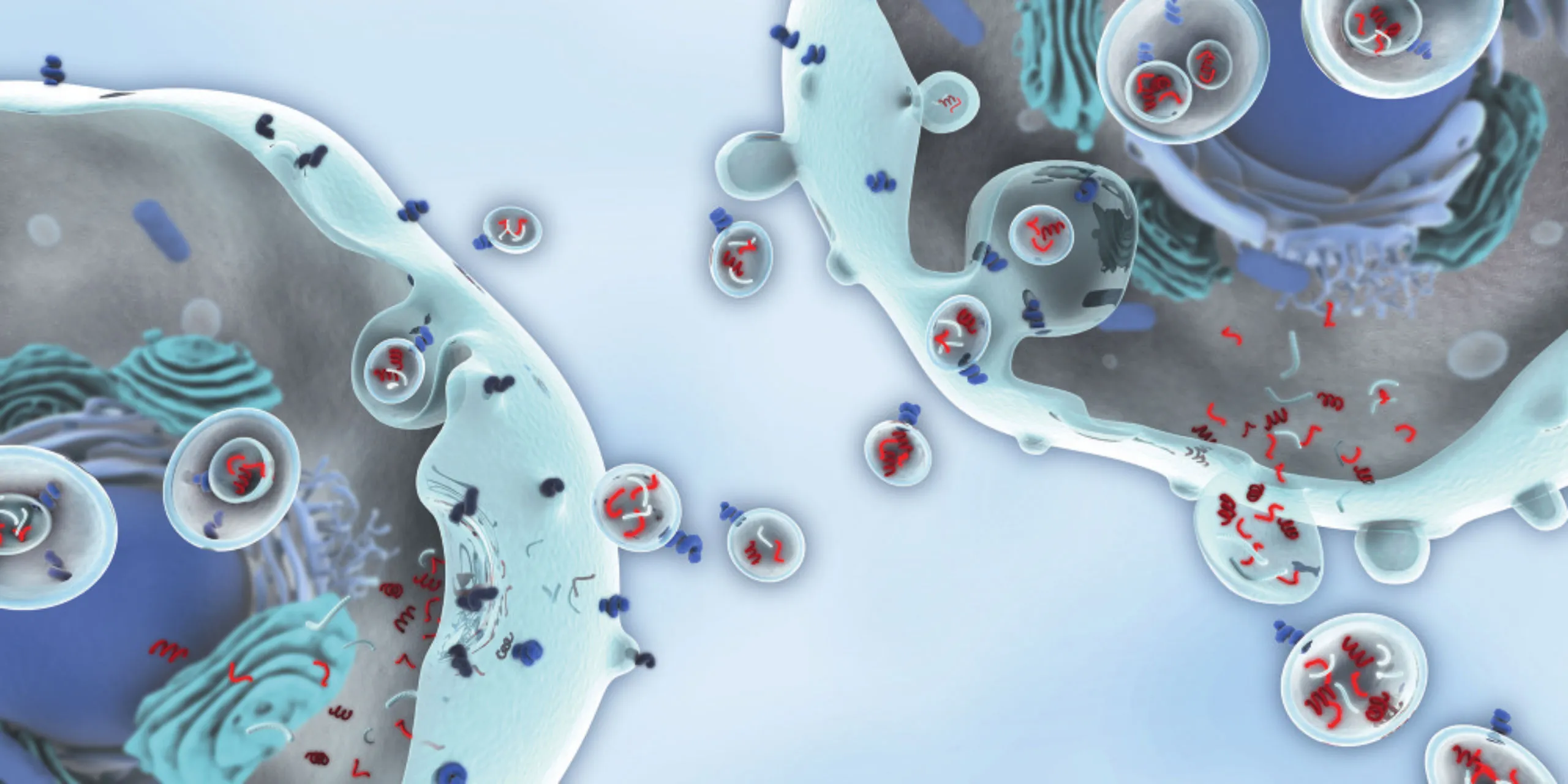
Small vesicle, strong cargo
Exosomes are 30–150 nm extracellular vesicles generated through the endosomal pathway and secreted when multivesicular bodies fuse with the cell membrane.Exosomes arise through a controlled intracellular process that determines their molecular cargo. This cargo includes proteins, microRNAs, messenger RNAs, lipids, and metabolites, making exosomes a molecular fingerprint of their parent cell.
The Medical Revolution in Miniature
The therapeutic potential of exosomes is transforming multiple fields of medicine. As natural drug delivery vehicles, they can cross biological barriers that synthetic nanoparticles cannot, including the blood-brain barrier. Their intrinsic biocompatibility and low immunogenicity make them ideal candidates for personalized medicine approaches.
Current applications under development include:
– Regenerative medicine: exosomes derived from stem cells promote tissue repair without the risks associated with cell transplantation
– Cancer therapy: engineered exosomes deliver targeted chemotherapy while minimizing systemic toxicity
– Neurodegenerative diseases: Modified exosomes transport therapeutic molecules across the blood-brain barrier
– Diagnostics: Exosome cargo profiles serve as liquid biopsy biomarkers for early disease detection
The ExoKnow Project: Engineering Excellence at Scale


ExoKNOW, a program co-funded by
European Regional Development Fund (ERDF)
Région Pays de la Loire
GenSensor’s team thanks the ERDF program team





